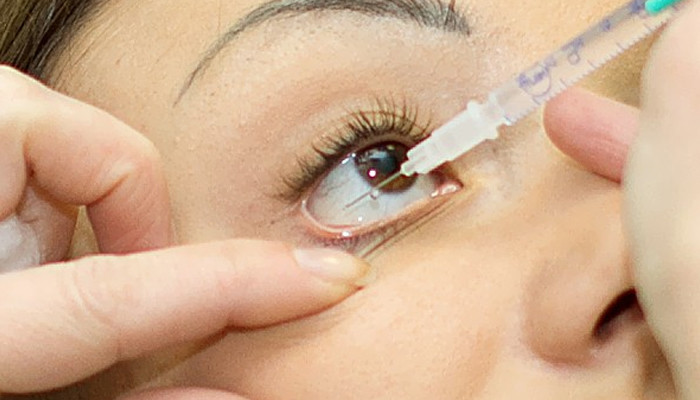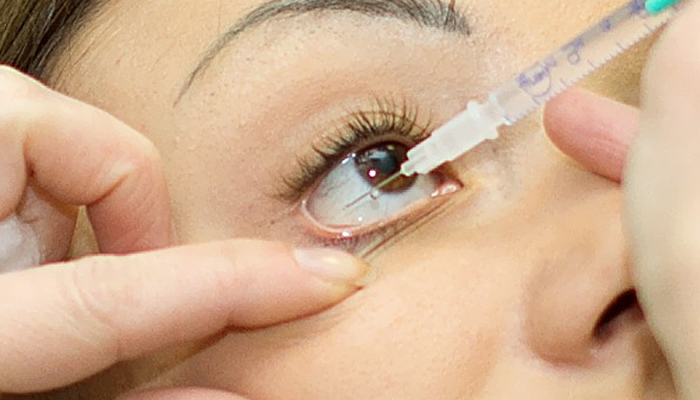
The Drug Regulatory Authority (DRAP) has banned hospitals and pharmacies across the country from using the injections after a case of eye damage from the injection came to light in Punjab.
The Drug Regulatory Authority has also stopped the sale of the relevant injections for the time being.
There are both unregistered and registered types of injections in the market according to Drip rules.
Drap warns that imported injections should not be used until quality checks are completed.
According to Drap officials, many hospitals across the country are engaged in repackaging this injection, no hospital, laboratory, pharmacy has a license to repack this injection.
The authorities say that patients should also avoid getting these injections for eye disease at present.
In this regard, ophthalmologists say that thousands of patients can be affected by not getting this injection.
Experts say that this injection is a cost-effective treatment for complications from diabetes, so Drip should license the drug to good institutions to make it available in the market.
setTimeout(function(){
!function(f,b,e,v,n,t,s)
{if(f.fbq)return;n=f.fbq=function(){n.callMethod?
n.callMethod.apply(n,arguments):n.queue.push(arguments)};
if(!f._fbq)f._fbq=n;n.push=n;n.loaded=!0;n.version=’2.0′;
n.queue=[];t=b.createElement(e);t.async=!0;
t.src=v;s=b.getElementsByTagName(e)[0];
s.parentNode.insertBefore(t,s)}(window,document,’script’,
‘https://connect.facebook.net/en_US/fbevents.js’);
fbq(‘init’, ‘836181349842357’);
fbq(‘track’, ‘PageView’);
}, 6000);
/*setTimeout(function(){
(function (d, s, id) {
var js, fjs = d.getElementsByTagName(s)[0];
if (d.getElementById(id)) return;
js = d.createElement(s);
js.id = id;
js.src = “//connect.facebook.net/en_US/sdk.js#xfbml=1&version=v2.11&appId=580305968816694”;
fjs.parentNode.insertBefore(js, fjs);
}(document, ‘script’, ‘facebook-jssdk’));
}, 4000);*/